Synthesis of Vinblastine Derivatives

- Authors: Hedvig Bolcskei, Lajos Szabo, Csaba Szantay3
-
View Affiliations Hide Affiliations3 Gedeon Richter Ltd. H 1475 Budapest, P.O.B. 27 Hungary, Institute of Organic Chemistry, Budapest University of Technology and Economics, H-1111 Budapest, Gellert ter 4, Hungary
- Source: Frontiers in Natural Product Chemistry: Volume 1 , pp 43-49
- Publication Date: January 2005
- Language: English
Preview this chapter:
Synthesis of Vinblastine Derivatives, Page 1 of 1
< Previous page | Next page > /docserver/preview/fulltext/9781608052127/chapter-5-1.gif
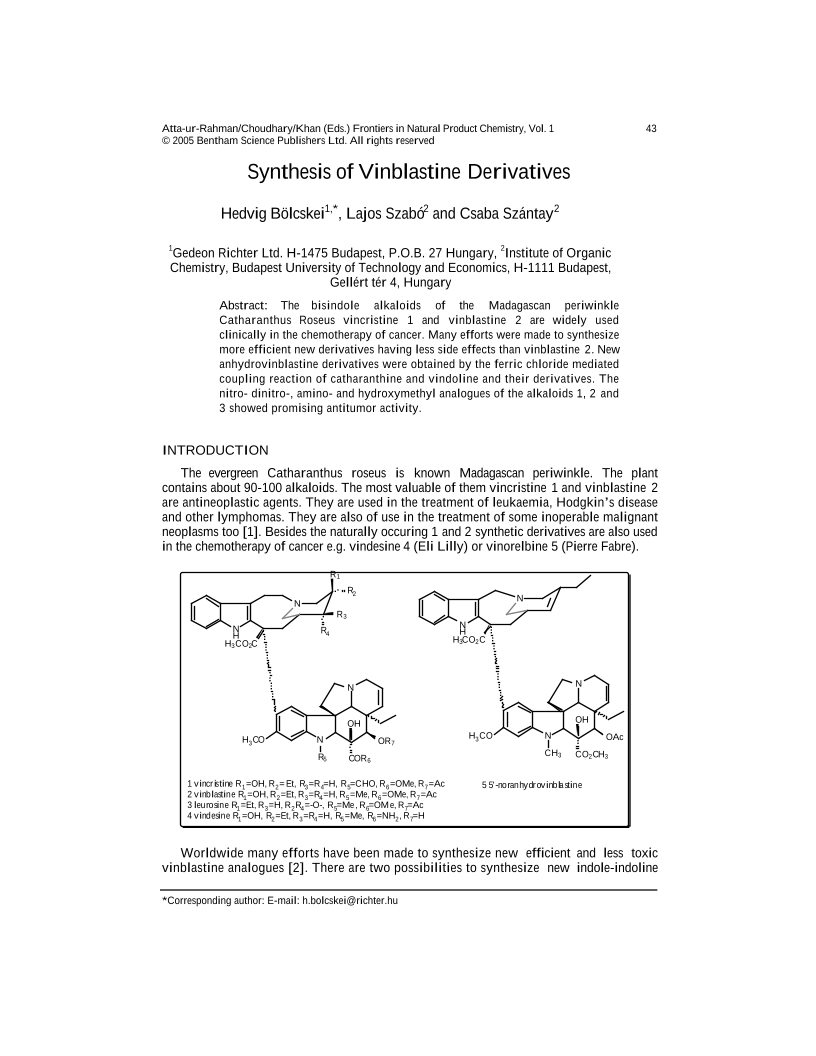
The bisindole alkaloids of the Madagascan periwinkle Catharanthus Roseus vincristine 1 and vinblastine 2 are widely used clinically in the chemotherapy of cancer. Many efforts were made to synthesize more efficient new derivatives having less side effects than vinblastine 2. New anhydrovinblastine derivatives were obtained by the ferric chloride mediated coupling reaction of catharanthine and vindoline and their derivatives. The nitro- dinitro-, amino- and hydroxymethyl analogues of the alkaloids 1, 2 and 3 showed promising antitumor activity.
Hardbound ISBN:
9781608056767
Ebook ISBN:
9781608052127
-
From This Site
/content/books/9781608052127.chapter-5dcterms_subject,pub_keyword-contentType:Journal -contentType:Figure -contentType:Table -contentType:SupplementaryData105
/content/books/9781608052127.chapter-5
dcterms_subject,pub_keyword
-contentType:Journal -contentType:Figure -contentType:Table -contentType:SupplementaryData
10
5
Chapter
content/books/9781608052127
Book
false
en

