Properties of Perhalopyridines
- Authors: Reza Ranjbar Karimi and Alireza Poorfreidoni
- Source: Perhalopyridines: Synthesis and Synthetic Utility , pp 1-7
- Publication Date: September 2020
- Language: English
Preview this chapter:

Properties of Perhalopyridines, Page 1 of 1
< Previous page | Next page > /docserver/preview/fulltext/9789811473791/chapter-1-1.gif
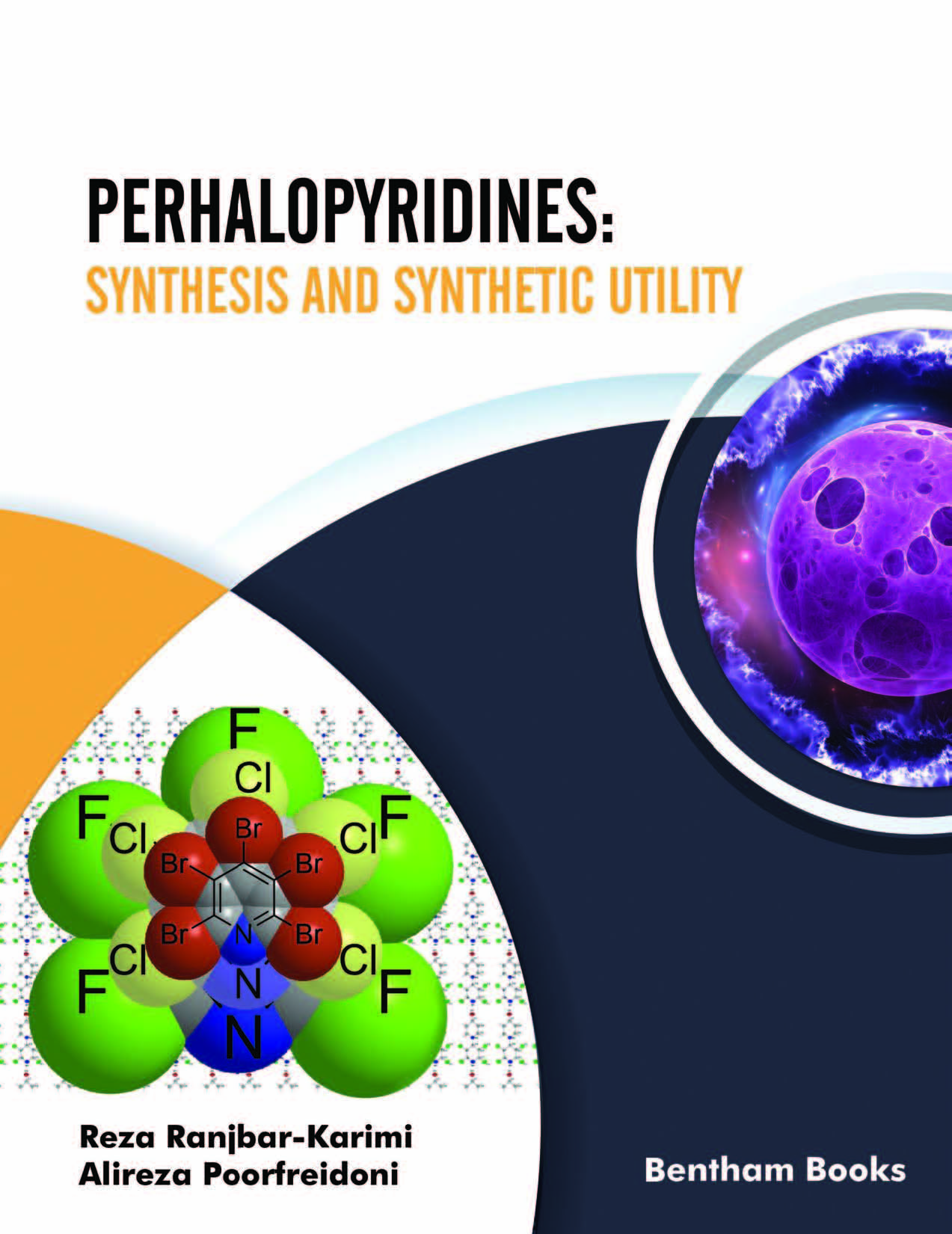
The introduction of halogen atoms on the pyridine ring causes significant changes in its properties. Halogens reduced basicity of pyridine ring as well as dipole moment. The presence of dense halogen atoms renders a higher density of perhalopyridines than pyridine. Fluorine atoms cause a low-field shift of pyridine carbons than chlorine and bromine atoms. Perhalopyridines are mainly involved in nucleophilic substitution reactions due to the electron-withdrawing nature of halogens while perfluoropyridines are more active than others.
Hardbound ISBN:
9789811473777
Ebook ISBN:
9789811473791
-
From This Site
/content/books/9789811473791.chapter-1dcterms_subject,pub_keyword-contentType:Journal -contentType:Figure -contentType:Table -contentType:SupplementaryData105
/content/books/9789811473791.chapter-1
dcterms_subject,pub_keyword
-contentType:Journal -contentType:Figure -contentType:Table -contentType:SupplementaryData
10
5
Chapter
content/books/9789811473791
Book
false
en

